Cost Effective
The number of actual sites participating would decline, which would reduce site setup, monitoring and associated costs
Increase and expedite patient recruitment
Increase patient diversity and enroll more participants from minority backgrounds.
Reduced patient dropouts
Automated remote digital data collection reduces drop-out rates by eliminating the need for frequent patient visits to the sites.
Track patient´s journeys in a single platform.
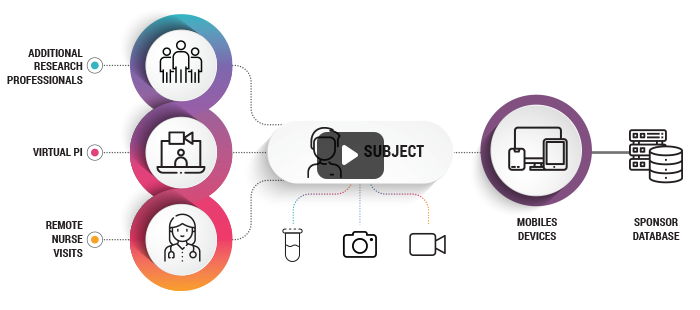
Digitizes The Clinical Trial Process For Patients, Sites and Sponsors
Enables Remote Trial Conduct and Supports Remote Site-Patient Interactions (Video & Text Chats)
Creates Audit Trails Of Every Interaction And Data Exchange
Real Time Data, Compliance and Status View Across All Relevant Roles
Facilitates Remote Monitoring Of The Clinical Trial

Patient eConsent
E2E consent workflow
Multimedia learning
Digital signature
Flexible configuration
Patient Engagement
Visits tracking
Medication compliance
Adverse events alerts
Motivate and Engage
Patient Data Capture
eDiaries / Questionnaires
Devices, Wearables
3rd Party data (e.g., Lab)
Surveys
ClinVigilant® Platform Capabilities
Digital consent management
Empowering patients
- Layered/Tiered consent
- Ability to withdraw consent anytime
Automated Reconsent management
With immediate alert as soon as new information is available
Tracking & document version management
Process administration flexibility
- Remote / at site
- Digital / paper process
- Electronic / Wet Ink signature
Educational information
For better comprehension of consent information (key word tagged)
- Glossary / Videos / Document / Images
Documentation:
Auto-generate eTMF related logs / forms
Knowledge and comprehension of the consent information
Omnichannel communication
For additional clarifications / information ask
- Text / Video chat
Visits management
- Visits overview
- Calendar integration
- Visit preparation information
- Scheduling and rescheduling site visits
- Alerts and Reminders
- Visit completion tracking
- Out of window visit alerts
- Unscheduled visits tracker
Trials medication management
- Reminders
- Consumption data collection
- Missed dose alerts
- Refill reminders
Tracking & document version management
Study related activities management
(e.g., diaries, PRO completion, dietary restrictions, sample collection) for optimum data quality
- Alerts and Reminders
- Completion tracking
- Missed activities alerts
Documentation
- Auto-generate eTMF related logs / forms
Trial specific education
- Consent related updates
- Current clinical trial updates
- General information on trials and related materials
Motivate and maintain compliance
- Site visit logistics management
- Gamification
- Status reports
- App messages
- Access to self health data
Secure omnichannel communication
- Text chat / Emails / In app notifications / Video chat
Information
- Standardised and tailor made communication plans
- Progress and performance assessment
- Care instructions
- Wellness and Prevention Guides
- Site team contact directory
- Services contact directory
- Nearest healthcare services e.g., pharmacy
Adverse Event
- Alert by patient to Investigator
Patient generated data
- Patient diaries
- Questionnaires including QoL
- Disease and behaviour management diaries
- Surveys
- Symptom assessments by self
- Images and videos
- End of trial surveys
Data capture
- Devices
- Apps
- Wearable
- Lab/3rd parties
Trials medication management
- Reminders
- Consumption data collection
Care coordination
- Between visits care Telemedicine
- Virtual assistance and coaching
- Real time direct data collection
- Early detection of abnormalities